Each day in companies across the globe, employees are being asked to do more with less. The mantra of the business community in the 21st century is “accelerating business”. You see it in marketing and all types of corporate communication. In the validation world accelerating system implementation, validation and deployment is the cry of every chief information officer and senior executive.
Accelerating validation activity means different things to different people. I define it as driving validation processes efficiently and effectively without sacrificing quality or compliance. Most manual processes are paper-based, inefficient, and cumbersome. Creating paper-based test scripts requires a lot of cutting/pasting of screenshots and other objective evidence to meet regulatory demands. Getting validation information into templates with proper headers and footers in compliance with corporate documentation guidelines is paramount.
The question for today is “HOW DO WE ACCELERATE VALIDATION WITHOUT SACRIFICING QUALITY AND COMPLIANCE?”
Earlier in my career, I developed “Validation Toolkit” for Documentum and Qumas. A Validation Toolkit was an early version of a validation accelerator. Many companies now have them. These toolkits come with pre-defined document templates and test scripts designed to streamline the validation process. They are called “tookits” for a reason – they are not intended to be completed validation projects. They are intended to be a starting point for validation exercises.
The FDA states in the General Principles of Software Validation; Final Guidance For Industry and FDA Staff issued on January 11, 2002, “…If the vendor can provide information about their system requirements, software requirements, validation process, and the results of their validation, the medical device manufacturer (or any company) can use that information as a beginning point for their required validation documentation…”
Notice, that the FDA says “… use a beginning point” for validation – NOT USE IT EXCLUSIVELY AS THE COMPLETE VALIDATION PACKAGE. This is because the FDA expects each company to conduct its own due diligence for validation. Also note that the FDA allows the use of such documentation if available from a vendor.
Beware that some vendors believe their “toolkits” can substitute for rigorous review of their software applications. It cannot. In addition to leveraging the software vendor information, you should ALWAYS conduct your own validation due diligence to ensure that applications are thoroughly examined without bias by your team or designated validation professional.
It is an excellent idea to leverage vendor information if provided. There is a learning curve with most enterprise applications and the use of vendor-supplied information can help streamline the process and add value. The old validation toolkits were delivered with a set of document templates and pre-defined test scripts often resulting in hundreds of documents depending on the application. In most cases, companies would edit the documents or put them in their pre-defined validation templates for consistency. This resulted in A LOT OF EDITING! In some cases, depending on the number of documents, time-savings could be eroded by just editing the existing documents.
THERE IS A BETTER WAY!
What if YOUR unique validation templates were pre-loaded into an automated Enterprise Validation Lifecycle Management system? What if the Commercial-off-the-Shelf (COTS) product requirements were pre-loaded into this system? Further, what if a full set of OQ/PQ test scripts were traced to each of the pre-loaded requirements? What if you were able to export all of these documents day one in your unique validation templates? What if you only had to add test scripts that represented the CHANGES you were making to a COTS application versus writing all of your test scripts from scratch? Finally, what if you could execute your test scripts on line and automatically generate a requirements trace matrix and validation summary report with PASS/FAIL status?
This is the vision of the CloudMaster 365™ Validation Accelerator. CloudMaster 365™ is the next generation of the validation toolkit concept. The ValidationMaster Enterprise Validation Management system is the core of the CloudMaster 365™ Validation Accelerator. The application can be either hosted or on-premise. This is a great way to jump start your Microsoft Dynamics 365® validation project.
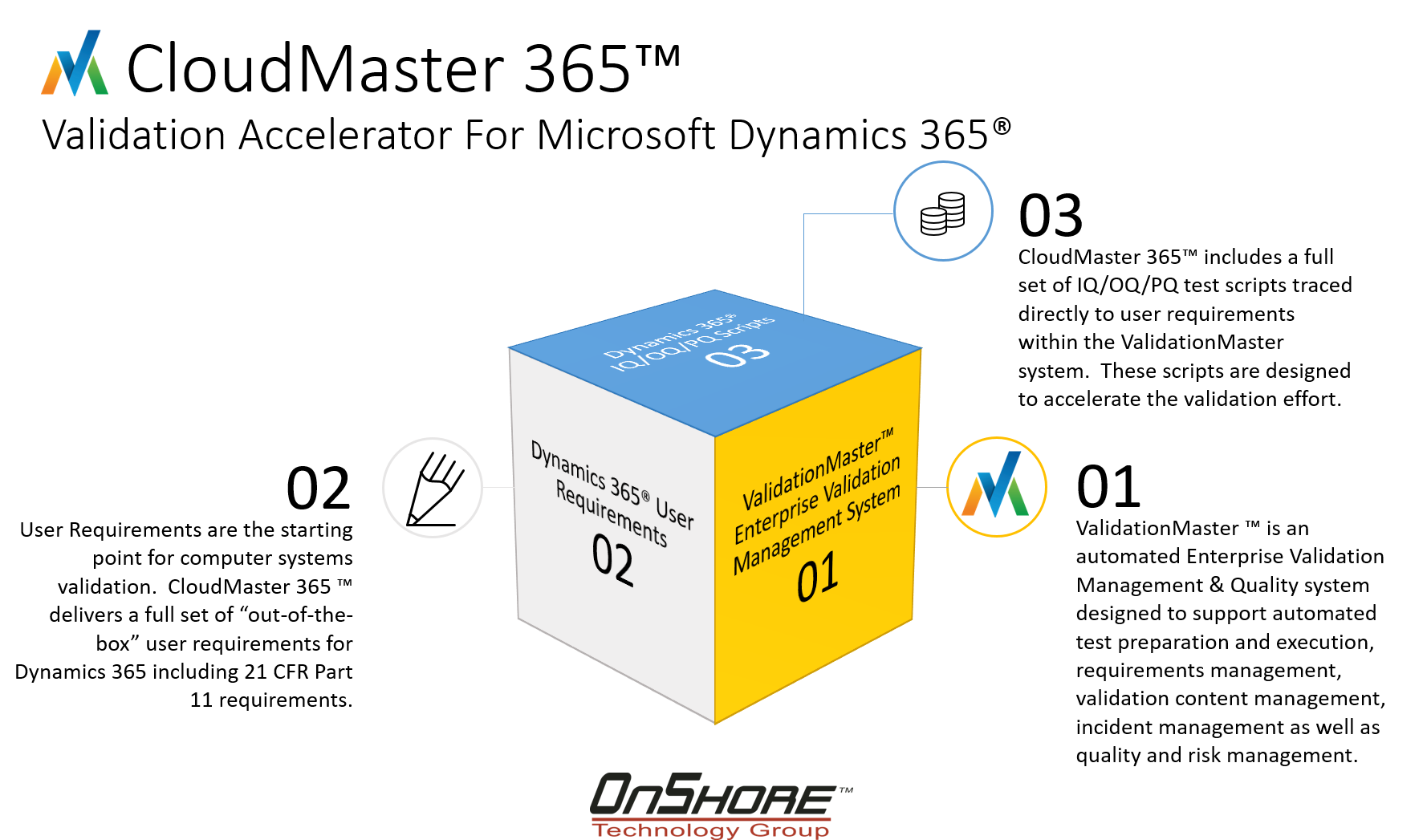
The CloudMaster 365™ Validation Accelerator includes (3) key components:
- ValidationMaster™ Enterprise Validation Management System
- Comprehensive set of user requirements for out-of-the-box features
- Full set of IQ/OQ/PQ test scripts and validation document templates
The Validation Accelerator is not intended to be “validation out of the box”. It delivers the foundation for your current and future validation projects delivering “Level 5” validation processes. If you consider the validation capability maturity model, most validation processes at level 1 are ad hoc, chaotic, undefined and reactive to project impulses and external events.
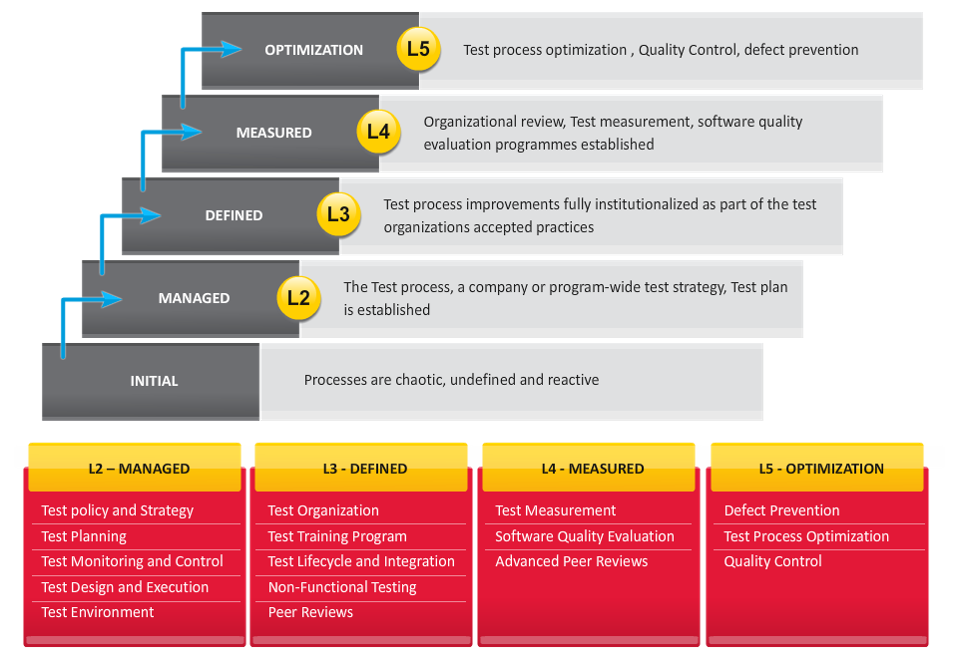
Level 5 validation is an optimized, lean validation process facilitated with automation. The test process is optimized, quality control is assured and there is a specific measure for defect prevention and management. Level 5 cannot be achieved without automation. The automation is powered by ValidationMaster™. With the CloudMaster 365™ strategy, instead of having “toolkit” documents suitable for only one project, you have a system that manages your COTS or bespoke development over time and manages the full lifecycle of validation over time as is required by regulators. This is revolutionary in terms of what you get.
CloudMaster 365™ TRULY ACCELERATES VALIDATION. It can not only be used for Microsoft Dynamics 365®, but is also available for other applications including:
- CloudMaster 365™ Validation Accelerator For Dynamics 365®
- CloudMaster 365™ Validation Accelerator For Merit MAXLife®
- CloudMaster 365™ Validation Accelerator For Yaveon ProBatch®
- CloudMaster 365™ Validation Accelerator For Oracle e-Business® or Oracle Fusion®
- CloudMaster 365™ Validation Accelerator For SAP®
- CloudMaster 365™ Validation Accelerator For BatchMaster®
- CloudMaster 365™ Validation Accelerator For Edgewater EDGE®
- CloudMaster 365™ Validation Accelerator For VeevaVault®
- CloudMaster 365™ Validation Accelerator For Microsoft SharePoint®
Whether you are validating an enterprise application or seeking to drive your company to higher levels of efficiency, you should consider how best to accelerate your validation processes not solution by solution but in a more holistic way that ensures sustained compliance across all applications. If you are embarking on the validation of Microsoft Dynamics 365®, or any of the applications listed above, the CloudMaster 365™ Validation Accelerator is a no-brainer. It is the most cost-effective way to streamline validation and ensure sustained compliance over time.







