Lean manufacturing has been with us since 1988. Lean principles were derived from the Japanese manufacturing industry. The “Lean” process was originally created and adopted by Toyota designed to eliminate waste and inefficiency in its manufacturing operations. Lean processes led to the Toyota Production System (TPS) which is arguably one of the greatest manufacturing success stories of all time. The focus of lean was the elimination of waste and inefficiencies throughout the manufacturing process.
To identify and eliminate waste from the production process, Toyota believed it was important to understand exactly the nature of waste and where it existed. While Toyota’s products significantly differed between factories, the typical wastes found in manufacturing environments were similar in nature. For each waste, Toyota developed an effective strategy to reduce or eliminate its effects, thereby improving overall performance and quality. The process became so successful that it has been embraced in manufacturing sectors around the world. Today’s manufacturers have embraced the concepts and philosophies of lean. Being lean is considered critical competitive advantage and strategic imperative. It has made Toyota an automotive success story.
I have been a validation practitioner for over 30 years. In the process of validating large engineering systems and life sciences quality and compliance management technologies, I have experienced first hand the waste involved in the validation processes. From manually cutting and pasting screenshots into test cases to manually tracing requirements to test scripts through manual document route/review processes and rewriting scripts for regression testing, waste abounds in the validation process. As I pondered my work over the past three decades, I began to think of a better way to validate computer systems.
In my initial research, I started to think of all of the wastes throughout validation processes and categorized them.
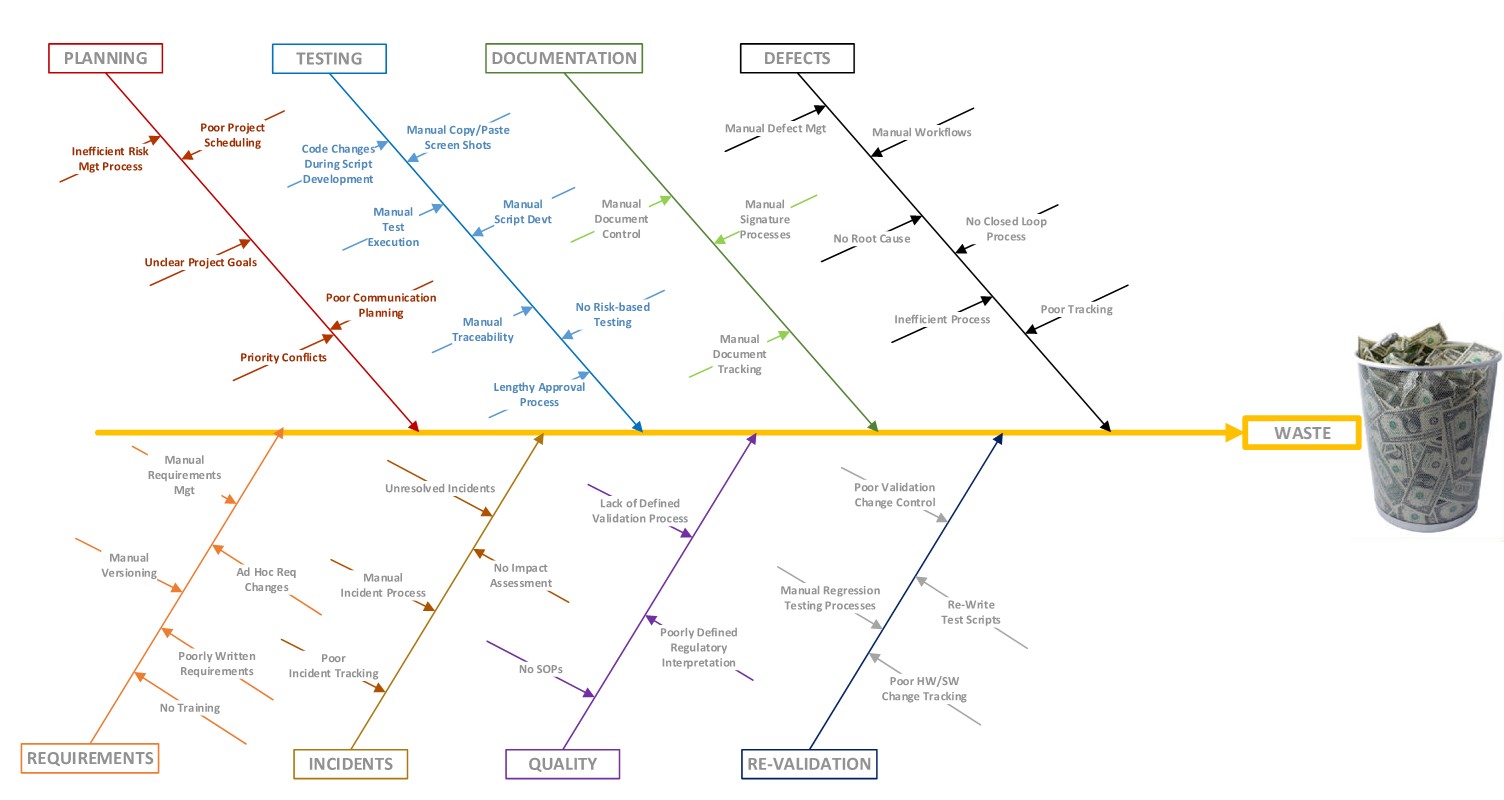
Wastes in the validation process include planning process wastes, testing wastes, documentation wastes, defect wastes, wasteful requirement processes, quality and incident management wastes and wastes associated with the re-validation and re-testing of software applications. As I began to ponder solutions to eliminate these wastes throughout the validation process, I embarked on the development of a Lean Validation strategy. We endeavor to save our clients both time and money throughout the validation process. OnShore Technology Group, Inc. is the only company whose practice focuses on the delivery of Lean Validation services. Our products and services are uniquely designed to power lean validation processes.
Using lean validation principles can result in the elimination of wasteful manual validation processes and significant improvements in validation efficiency, document cycle times, increased testing productivity and greater ability to identify and correct software defects leading to enhanced software quality, lower costs and improved regulatory compliance.
OnShore Technology Group, Inc. has pioneered the principles and best practices of “Lean Validation” – the process of eliminating waste and inefficiencies while driving greater software quality throughout the validation process. In addition to the delivery of expert lean validation services, OnShore’s flagship software application is known as ValidationMaster™ – the FIRST Enterprise Validation Management and Quality system designed to automate lean validation processes. ValidationMaster™ delivers a single source of truth for any type of validation including software, equipment, process, cold chain, facility, and other types of validation projects.
ValidationMaster™ is also the first validation management system accessible via any Window, Apple or Android mobile device. The system includes key features such as a validation dashboard, fully automated test script development and execution, automatic requirements traceability, custom report development and generation and a full range of quality management capabilities (training, audit management, change management, controlled document management, ISO, validation KPI’s, CAPA, nonconformance management and much more.
In 2017, OnShore Technology Group, Inc. was recognized as the “Best IV&V Automation Solutions Provider” and the “Software Validation Testing Experts of the Year”. In 2016, OnShore made the coveted annual list of CIOReview Magazine as one of the 20 Most Promising Pharma & Life Sciences Tech Solution Providers”.
Contact us today to learn more and see a live demonstration of ValidationMaster™.








1