For today’s on-premise and cloud-based systems, validation testing is a required process to ensure that systems are of sufficient quality and operate according to their intended use. Validation testing is typically done at the end of the development process after all verification has been completed. IEEE defines validation as the process of evaluating software to determine whether it satisfies the specific defined requirements. Therefore validation testing must be traced to pre-defined requirements.
The goals of validation are pretty clear:
- Discover errors/anomalies in software prior to production
- Confirm that system meet their intended use
- Confirm that regulatory requirements in the software are met
- Provide due diligence (documented evidence) for regulators
- Deliver justification for use of a system
I have had the priviledge of working with many life sciences companies over the years and I have seen it all – from ad hoc testing processes to those that are well-defined and mature in their optimization and effectiveness. Most testing processes are at level one where the processes are chaotic and not well-defined.
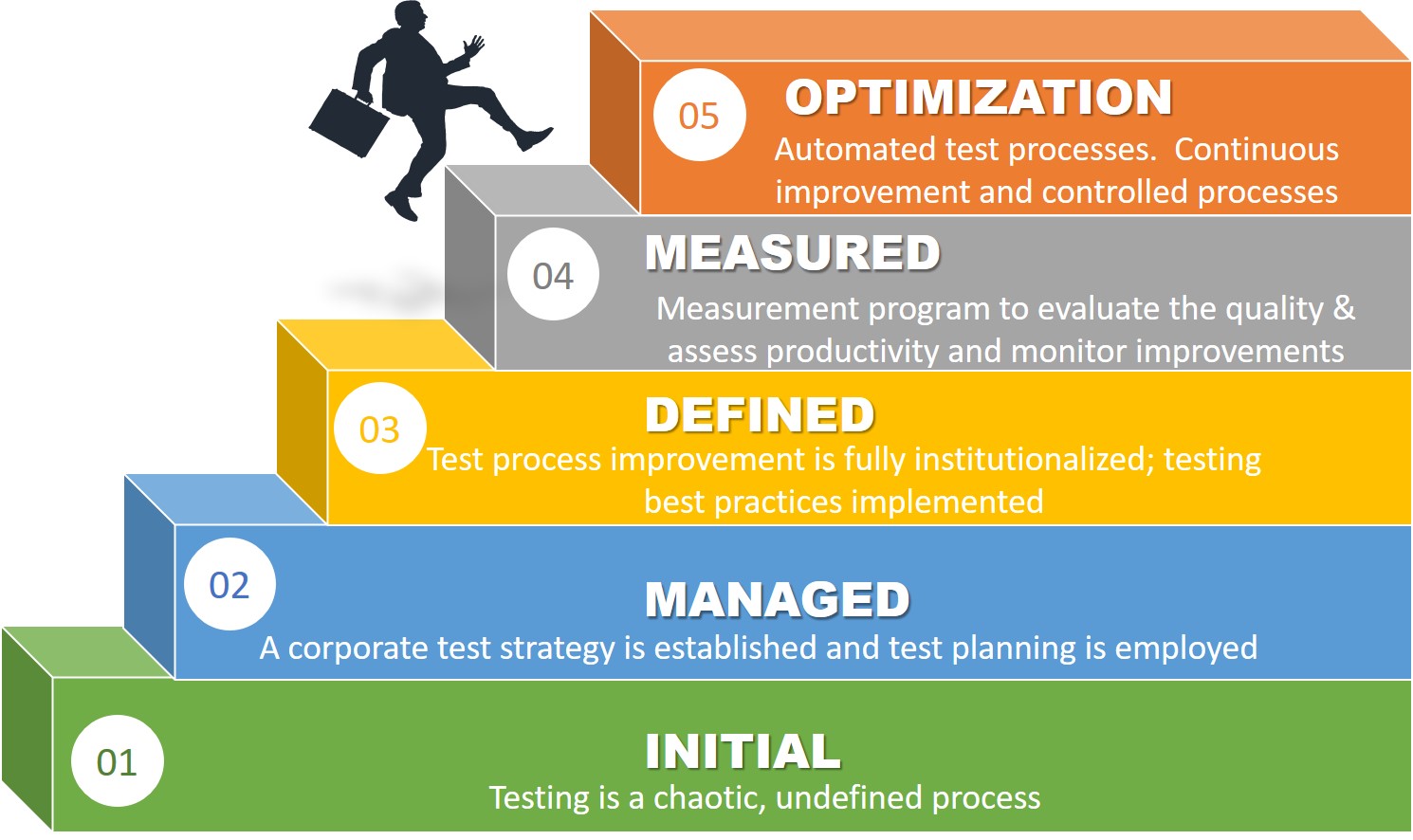
Automated validation testing processes are essential in today’s life sciences companies where we all are being asked to do more with less. It is essential that we establish automated processes to accelerate productivity, eliminate waste and ensure greater to ensure software quality.
The less time spent on the mechanics of test script development, the more time can be dedicated to ensuring software quality.
The software testing capability maturity model should be on your radar. Establishing automated testing should be a goal for every validation engineer. It is important to understand how to achieve Level 5 and what it takes from a process perspective to achieve greater testing governance and sustained compliance.
ESTABLISHING A REUSABLE TEST SCRIPT LIBRARY
When conducting validation, the most laborious part of the process is testing. Validating today’s COTS software applications involves testing the same “out-of-the-box” features over and over again. Many validation engineers continue to draft test scripts again and again to support this process. What if you could establish a “reusable test script library” for your validation projects that would allow you to conduct regression testing quickly and easily without major rewrite for your applications? What if you could centrally store this repository for all of your applications so you had a single source of truth for all of your validation projects? What if you could ensure that your validation test library was “auditable” and could be shared with regulators during audits as part of your objective evidence requirements? What if each test script had its own audit trail and was traced to its respective requirements for automatic traceability?
The ability to effective establish and manage a reusable test script library and a single source of truth for all of your validation projects is made possible with the ValidationMaster™ Enterprise Validation Management system.
The system allows you to create, track and manage a reusable test script library quickly and easily. All of your validation assets are in a single location for reference and reuse. Intelligence can be quickly gleaned from the system to drive continuous improvement and compliance. For fully automated scripts that require no human intervention to run, the system has the ability to automate test script execution and reporting of actual results. This helps to facilitate continuous testing in the cloud and ensure that your systems are maintained in a validated state.
Validation testing is here to stay. AUTOMATION IS THE KEY! It is a necessity not a luxury to automate your validation processes. Join us for one of our Automated Testing online web briefings to learn more.








1